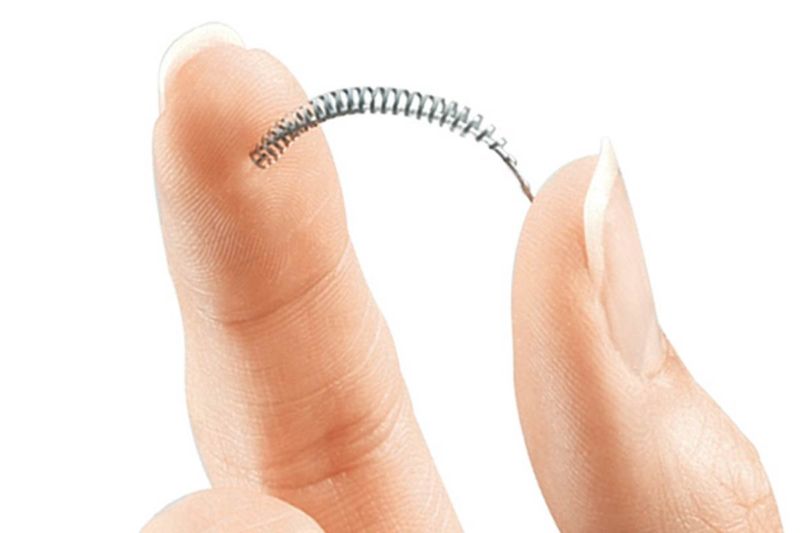
Essure, manufactured by Bayer, is meant to be a noninvasive way to permanently prevent a woman from becoming pregnant. Doctors insert a small metal coil in each fallopian tube, and the scar tissue that forms around the coil seals the tube shut. Thousands of women across the country allege these devices are defective and/or caused serious complications they were never warned about.
If you believe you suffered complications or injuries because of an Essure Permanent Birth Control System implant, you may be eligible to file a claim for compensation against Bayer. An Essure birth control complication lawsuit class action lawyer at Gacovino, Lake & Associates, P.C. can help. We can help you understand your legal options, and will fight for the compensation you deserve if you suffered damages because of this device.
Call our office today at 631-600-0000 to schedule your complimentary consultation.
What Are the Most Common Complications Related to Essure?
Essure first hit the market in November of 2002. Since that time, the U.S. Food and Drug Administration (FDA) has received thousands of reports about complications related to this device. The FDA reports it received a total of 14,919 between 2002 and the end of 2016.
The most common complaints of symptoms include:
- Abdominal pain and cramping
- Heavy bleeding and menstrual irregularities
- Headache
- Fatigue
- Weight fluctuations
Some of the women who are pursuing lawsuits against Bayer allege more serious complications related to the Essure birth control system. These complications include:
- Severe pain and bleeding
- Migration of the devices
- Organ perforation
- Birth defects in children born after placement
More specifically, the FDA received:
- More than 2,400 reports of allergies or other incompatibility issues
- Almost 1,500 reports of device migration
- More than 650 reports of “device operating differently than expected”
- Reports of 617 broken devices
Any of these complications can disrupt a woman’s life greatly. She may require hospitalization or prescription medications, miss work, be unable to care for herself or her family, and in some cases even require surgery.
Has the FDA Issued a Warning About Essure?
Despite the wealth of complaints against the Essure Permanent Birth Control System, the FDA has not taken any action to remove it from the market or add any significant warnings about the product. It did, however, require a boxed warning on these devices that explains the potential side effects and complications. It hopes this allows women to make a more educated choice about implantation of this device.
While the FDA says the benefits of these devices outweigh the risks for women seeking this type of birth control option, they are continuing review reports of complications and monitor developments in research related to the device. This includes an ongoing post-market surveillance study to collect clinical data about the frequency and severity of complications suffered after the placement of these devices.
What Is the Status of Essure Birth Control Complication Lawsuits?
When women first began pursuing legal action against Bayer because of the Essure devices, they ran into a major road block. Essure received the FDA’s pre-market approval, meaning it had to undergo rigorous testing and clinical trials before receiving the go-ahead.
The FDA allegedly reviewed the design, manufacture, instructions, and even the box warnings for the product before it ever hit the market. This type of pre-market approval bars individuals from filing civil suits against the drug or device manufacturer under preemption laws, since the item has already undergone such strict scrutiny and the FDA deemed it safe. This preemption status made it difficult to file a claim against Bayer, despite the injuries suffered by these women.
Even still, Bayer stated in early 2017 that it has faced 3,700 lawsuits.
They allege Bayer acted negligently in manufacturing the devices, adequately managing risks, and training doctors to use the devices properly. In addition, other allegations include fraudulent manufacture and breach of express warranty.
What Should I Do If I Suffered Complications Caused by an Essure Permanent Birth Control System?
It is not too late to collect compensation to cover your medical bills, lost wages, pain and suffering, and other related damages if you suffered complications because of an Essure device implant.
At Gacovino, Lake & Associates, P.C., our skilled and knowledgeable birth control complication lawsuit class action lawyers will listen to your story and determine if you have a case. We can determine if you are eligible, and file your case if so. We are not afraid to go toe-to-toe with Bayer or any other pharmaceutical company. We will fight for the full value of compensation you deserve, and you pay us nothing until we recover money for you.
Call us today to learn if you are eligible to file a claim based on your experience with the Essure Permanent Birth Control System. We are available now at 631-600-0000.